Recalls On Ceramic Hips

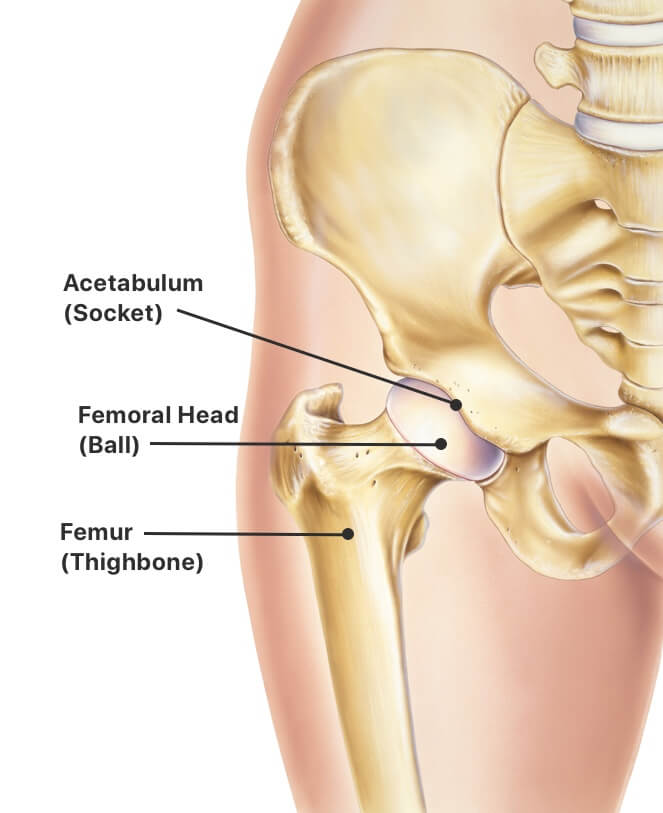
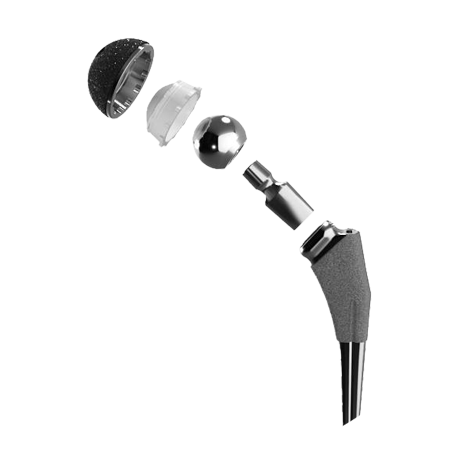
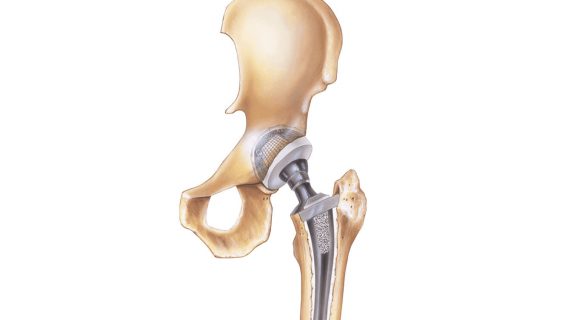
Polyethylene a type of plastic ceramic or metal.
Recalls on ceramic hips. The pinnacle hip system is constructed of metal ceramic and polyethylene but the metal lining and metal femoral head have been linked to. The trident ceramic acetabular system fda approved in 2003 was a ceramic on ceramic hip implant that promised less wear and tear than plastic or metal components. A number of surgeons and researchers provided data on metal on metal hips. In february 2001 the fda announced that biomet us manufacturer of artificial hips had made a voluntary recall of its hip replacement system following discovery of defects in the zirconia ceramic femoral heads.
Many of biomet s artificial hips are made of metal also known as metal on metal hips. Two years later the 93 000 people worldwide who had the metal on metal hips implanted were notified of unusually high failure rate as high as 49 failure within six years and a subsequent recall. Food and drug administration fda may be made of one or more of three different materials. Thrs place metal ceramic or polyethylene implants in the top of the femur and the pelvic hip socket.
Following the massive depuy recall of metal on metal hip implants ceramic hips are fast becoming the implant of choice. A durable titanium sleeve would protect the ceramic from chipping and fracturing the company said. Ceramic hip implants are designed to be the most scratch resistant and smoother which means that wear better than all of the hip replacement implants. Food and drug administration fda took action to investigate.
In 2012 amid numerous reports of metal on metal hip failures more than with other types of hips the u s. More than 600 000 hip replacement surgeries were performed in the united states between 2012 and 2018. There was a lot of hope for these metal on metal hips but many of them turned out to cause more harm than good and led to a number of hip replacement recalls. The hearing was open to the public.
The neck components of the rejuvenate and abg ii are made of chromium and cobalt and the stems are coated with titanium. Once recovered from surgery thrs should ideally leave the patient with improved mobility and less pain and stiffness. Artificial hip implant systems that are approved by the u s. Since a large number of recalls involved metal on metal hips the fda called a panel meeting in june 2012 to discuss issues related to these devices.
Ceramic hip replacement implants also use metal parts that fit within the bone but the bearing surface the ball and the socket can be made of the ceramic material.